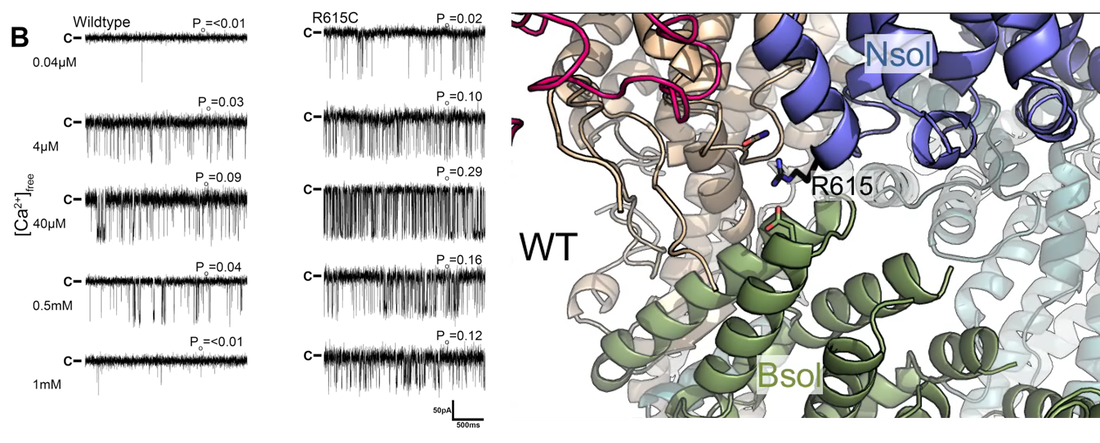
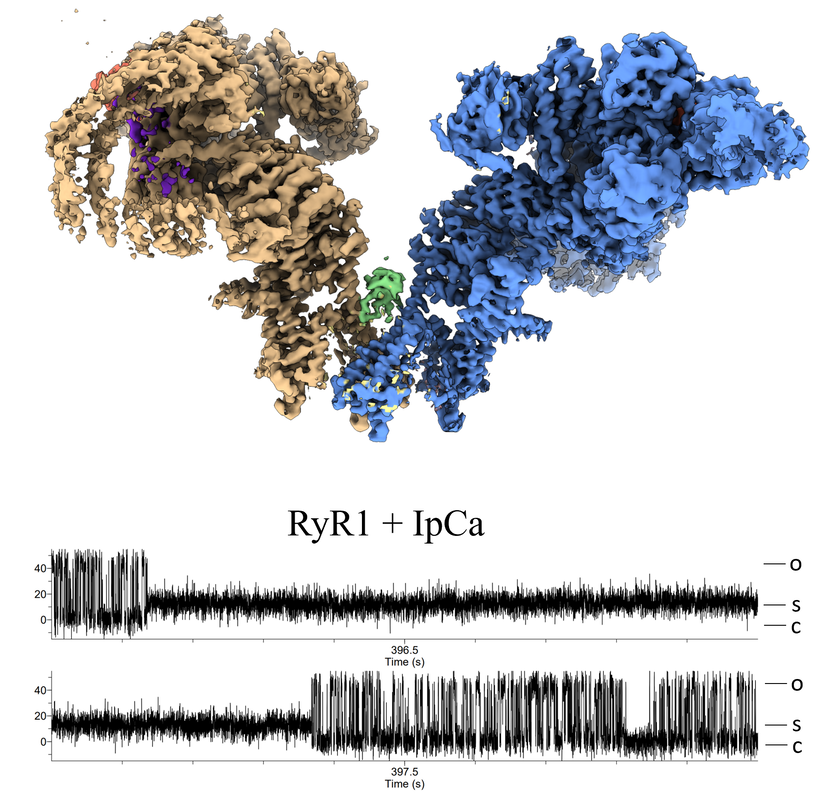
From heartbeat to neuronal signals: ion channels in health and disease |
The University of British Columbia (UBC)
Department of Biochemistry and Molecular Biology
Life Sciences Institute
Department of Biochemistry and Molecular Biology
Life Sciences Institute